N-aryloxide-amidinate group 4 metal complexes
Hanhua Xu, Ze-Jie Lv, Junnian Wei*, Zhenfeng Xi
Dalton Trans., 2023. DOI: 10.1039/D3DT01767B.
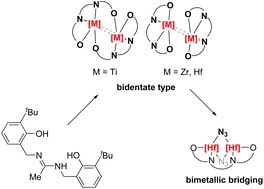
A N-aryloxide-amidine ligand (H3L), integrating phenoxide (PhO−) and amidine ligands through methylene linkers, has been synthesized from 2-(aminomethyl)-6-(tert-butyl)phenol in two steps. Upon reacting the deprotonated H3L ligand with group 4 metal chloride MIVCl4, a corresponding (LMIV–Cl)2 dimer could be obtained. The coordination modes exhibit variation depending on the radius of the metal ions. In the case of (LTiIV–Cl)2, the two ArO− arms from the same ligand bond to two different Ti(IV) centers, while in the case of (LZrIV/HfIV–Cl)2, both ArO− arms coordinate with the same metal center. Moreover, the two C–N bonds in the amidinate moiety are localized in (LTiIV–Cl)2, whereas they delocalize in (LZrIV–Cl)2. Notably, (LHfIV–Cl)2 could further react with one equivalent of HfCl4, yielding the binuclear metal azide in the presence of KN3 and LiCl, where the coordination mode of the amidinate moiety changed from the bidentate chelating type to the bimetallic bridging coordination.